
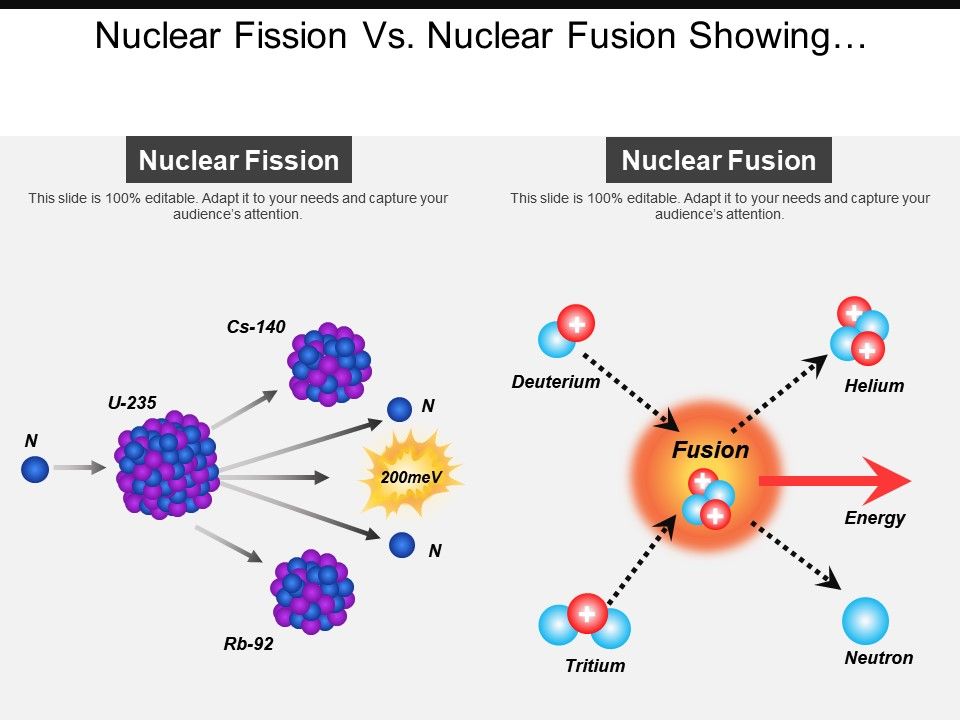
This figure is dated and probably high, but it gives a basis for comparison. Nuclear fission is the splitting of a heavy nucleus into two lighter ones.
Nuclear fusion and fission interactive code#
Then they are expressed in terms of a nominal per capita U.S. In this paper the analysis of water activation calculation with taking the reaction cross section uncertainties into account is performed with the RRUNC code 9.MCNP 8 was used to calculate neutron spectra in the first wall cooling system for fusion reactors and in the reactor core in a fission reactor. Both the single event energy and the energy per kilogram of fuel are compared. Nuclear binding energy curveĭeuterium-tritium fusion and uranium-235 fission are compared in terms of energy yield. Its average binding energy per nucleon is exceeded only by 58Fe and 62Ni, the nickel isotope being the most tightly bound of the nuclides. Iron-56 is abundant in stellar processes, and with a binding energy per nucleon of 8.8 MeV, it is the third most tightly bound of the nuclides. The iron limitThe buildup of heavier elements in the nuclear fusion processes in stars is limited to elements below iron, since the fusion of iron would subtract energy rather than provide it. Whereas an atomic transition might emit a photon in the range of a few electron volts, perhaps in the visible light region, nuclear transitions can emit gamma-rays with quantum energies in the MeV range. The binding energies of nucleons are in the range of millions of electron volts compared to tens of eV for atomic electrons. The fact that there is a peak in the binding energy curve in the region of stability near iron means that either the breakup of heavier nuclei (fission) or the combining of lighter nuclei (fusion) will yield nuclei which are more tightly bound (less mass per nucleon). The binding energy curve is obtained by dividing the total nuclear binding energy by the number of nucleons. The nuclear binding energies are on the order of a million times greater than the electron binding energies of atoms. The comparison of the alpha particle binding energy with the binding energy of the electron in a hydrogen atom is shown below. The enormity of the nuclear binding energy can perhaps be better appreciated by comparing it to the binding energy of an electron in an atom. It also showcases interactive models of the first atomic bombs and simulation of the Nuclear Winter effect.

This binding energy can be calculated from the Einstein relationship: Nuclear binding energy = Δmc 2įor the alpha particle Δm= 0.0304 u which gives a binding energy of 28.3 MeV. This collection contains animations of a nuclear chain reaction, nuclear fission and nuclear fusion.

The difference is a measure of the nuclear binding energy which holds the nucleus together. Nuclei are made up of protons and neutrons, but the mass of a nucleus is always less than the sum of the individual masses of the protons and neutrons which constitute it. Nuclear Binding Energy Nuclear Binding Energy


 0 kommentar(er)
0 kommentar(er)
